Introduction
Thermodynamics, as with most concepts in science and physics, is most accurately described using mathematical equations. However, simple explanations, although not perfectly accurate, are sufficient to convey the information needed to understand everyday experiences. This paper is an attempt to simplify these concepts.
Thermodynamics simply describes the movement of heat. Thermodynamics is derived from thermo, meaning heat, and dynamics, (literally “power”), and is used to describe the movement or change of a process due to heat flow.
Heat and temperature are often confused or used interchangeably. Heat is the flow of energy from one object or system, to another object or system. Temperature is a measure of the internal kinetic energy of an object. As an example, a frying pan has a high temperature because the molecules of the metal are moving quickly. When an egg is cracked into the pan, heat flows from the pan to the egg. Although not obvious, the temperature of the pan will drop slightly as it transfers heat to the egg.
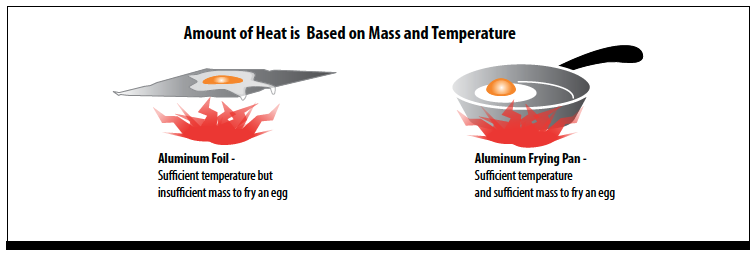
The amount of heat an object has is based on its mass as well as its temperature. Although they may be the same temperature, aluminum foil cannot store enough heat to cook an egg, while an aluminum frying pan, with its greater mass, can.
Cold is the absence of heat
Cold is “made” by removing heat from an object or system. Cold is not a substance, as heat is not a substance. Both are just descriptions of the movement of energy from one place to another. Heat will always move from a hot material to a cold one. Hot things cool because the heat moves out of the material, cold things warm because heat moves into the material.
Heat can move in three ways; conduction, convection and radiation.
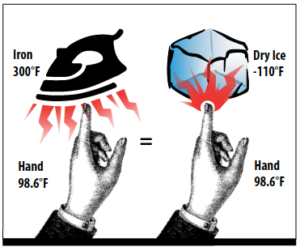
Conduction is the easiest and most familiar to anyone who has burned themselves on a stove or a hot pot. It is the direct transfer of heat from one object to another, when they are in contact. Since heat is the flow of energy, and it is driven by the temperature difference between two objects, an iron at 300° and a piece of dry ice at -110° will both burn a finger because the temperature difference between the finger and the iron or dry ice is about the same. In one case the flow of heat is into the finger, in the other the flow of heat is out of the finger, but in both the heat flow is high. See Figure 2.
Convection is based on the movement of a fluid to transfer heat from one object or area to another. Weather is a convective process, hot air and warm water move from the tropics toward the poles.
Radiation is heat transferred directly from a source to an object without using a medium like air or water. Heat radiated from the sun will melt snow on the road, even on a cold day. The radiant energy warms the road surface without directly warming the air.
In the real world, the transfer of heat involves a mixture of all three methods, with the possible exception of outer space. A puzzle the reader may enjoy is “what is the temperature of outer space?”. The answer will be found at the end of Part 2.
Moving heat from one object or area to another allows comfort heating and air conditioning, as well as refrigeration for food preservation and processes.
Definitions
States of matter There are three common states of matter*, solid, liquid and gas.
*Plasma is another state of matter but is beyond this discussion.
A solid will maintain its shape (ice, wax, steel), while a liquid will flow and take the shape of its container (water, mercury). A gas or vapor will fill all available volume (steam, air, mercury!).
States of matter are generally based on the form of the substance at room temperature and pressure. Things like air are gaseous at room temperature while water is a liquid. When heated, water become gaseous, but is referred to as a vapor.
It may be surprising that things we generally experience as solids or liquids can exist in other states as well. A small drop of mercury liquid is vaporized in a fluorescent light bulb and causes the bulb to “fluoresce”. Even a solid metal like gold or aluminum can be vaporized and the vapor used to cover plastic or other materials. The DVD (Direct Video Disk) you watch movies on may be made by DVD (Direct Vapor Deposition), or sputtering, which uses gold vapor to plate the surface.
Liquid, vapors and gases are classified as fluids because they can flow. Electricity can also be classified as a fluid because it flows in circuits. Please refer to KE2 Therm Bulletin 9.W.4 Electronics 101 for more information.
Phase changes- When a substance changes from one state of matter to another, it experiences a phase change. For instance, ice melting into water is a phase change from solid to liquid. Water changing into steam is a phase change from liquid to vapor. Energy in the form of heat is required to create a phase change and is the basis of most refrigeration and air conditioning.
BTU- British Thermal Unit- the amount of heat energy required to raise one pound of water one degree Fahrenheit.
A Ton of refrigeration or air conditioning is the amount of heat needed to melt one ton of ice in 24 hours. The amount is 12,000 BTUs per hour.
MBH- thousands of BTUs per hour. Used as a method to simplify quantities. 340 MBH is easier to use in specifications than 340,000 BTUs per hour.
Energy is the measure of the ability to do work. There are a number of types, including thermodynamic (heat), potential, kinetic, sound, light, electromagnetic, chemical and nuclear.
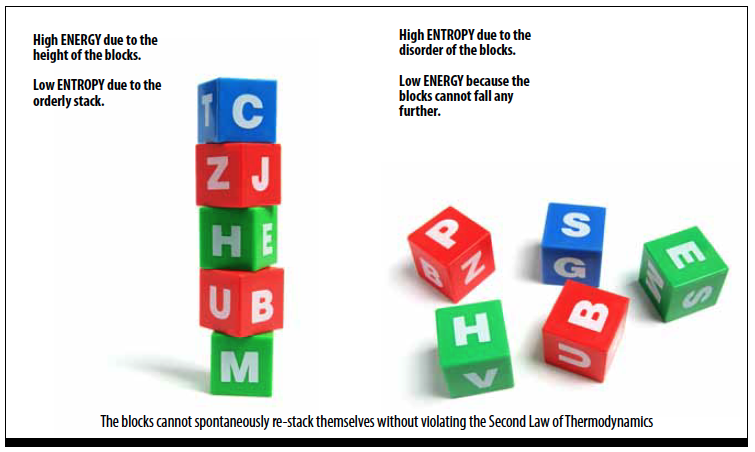
Entropy is the measure of the disorder of a system. Sometimes defined as the energy of a system that is not available to do work.
Systems decrease in energy and increase in entropy over time. The process is not reversible. For example, a stack of blocks falling over will result in a lower energy state and higher system entropy. See Figure 3.
Temperature Scales are used to measure temperature, not directly heat.
F Fahrenheit is based on the properties of water. Water freezes at 32˚F and boils at 212˚F . Fahrenheit is used in the “English” system.
C Celsius was called Centigrade, and is based on the properties of water. Water freezes at 0˚C and boils at 100˚C. , Celsius and Kelvin are used in the Metric or SI (Standard International) system
K Kelvin is based on Absolute Zero. Absolute Zero is zero K. A degree of Kelvin is the same as a degree of Celsius. Kelvin notations do not contain the degree (˚) sign.
Absolute Zero is the theoretical temperature at which all motion, including molecular motion, ceases. It is not physically possible to reach without violating the Third Law of Thermodynamics
0 Kelvin, -273.15˚ Celsius, -459.67 ˚ Fahrenheit
Three Laws of Thermodynamics
Traditionally, three laws of thermodynamics are used, but there is another. This is called the “Zeroth”Law and states, in a rather complicated way, that no heat will flow between two objects that are at the same temperature. This seems so obvious that this law is generally ignored, in common practice. In addition, and to add further complication to the mathematical statements of the laws, is the frame of reference. The laws hold for closed systems where no energy can be brought in from an external source. Open systems have different characteristics. Since the universe is considered a closed system, the laws are valid.
The Three Laws in Their Simplest Form
First Law – Energy cannot be created or destroyed, but can change form, and location. For instance, burning wood changes the internal energy in the wood into heat and light energy.
Second Law – The Second Law is the most understandable and useful in real world applications, and makes heating, air conditioning and refrigeration possible. Energy must flow from a higher state to a lower state. That is, heat must always flow from the warmer object to a cooler object and not from the cooler object to the warmer object.
The Second Law holds in our everyday visible world, but on the subatomic level the law is constantly violated, but statistically the law holds true. Although beyond the scope of this paper, an interested reader will find fascinating theories in quantum mechanics. A search of the “arrow of time” will yield interesting variations of the Second Law.
Third Law- As a system approaches absolute zero, the entropy of the system approaches a minimum value. Absolute Zero cannot be attained in a real system, it is only a theoretical limit.
