An appreciation of the terms superheat and saturation is the key to understanding and troubleshooting air conditioning and refrigeration systems. Illustrations in this document show closed vessels for simplicity, but real systems consist of heat transfer surfaces (condenser and evaporator), a compressor and a restriction device.
Goals
By the end of this lesson you will understand superheat, saturation, subcooling, temperature glide and how they pertain to air conditioning and refrigeration systems. Azeotropic refrigerants will also be explained.
Definitions
Figure 1 - Fluid in a Saturated Condition
Saturation means that, in a closed container containing a single fluid, the liquid and vapor have reached thermodynamic equilibrium. For this discussion, a single fluid may be a mixture of fluids that act as one fluid. See azeotrope, further on. An easier way to say, and understand this, is that the number of molecules evaporating is the same as the number of molecules condensing back into liquid, regardless of the temperature. At equilibrium, the pressure of the mix will be directly proportional to the temperature of the mix. See Figure 1.
Figure 1 - Fluid in a Saturated Condition
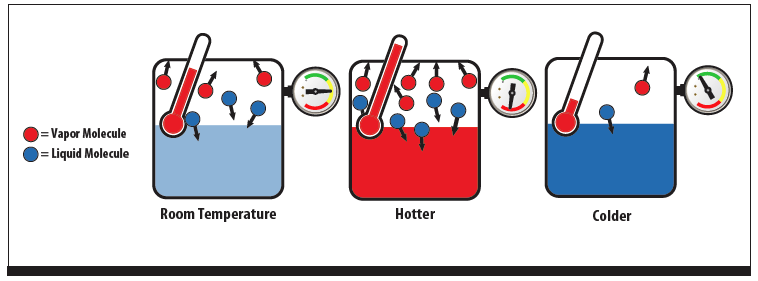
In the hotter instance, the pressure will increase because more molecules are hitting the inside of the container. As long as liquid and vapor are present in the vessel at the same time, the fluid will be saturated and follow this rule. An everyday example is the propane tank on a gas grill. A pressure gauge on the tank will not indicate how full the tank is, only the pressure, and therefore, the temperature of the propane. The temperature pressure chart Table 1, shows this relationship. Once all the liquid propane is used, the pressure will begin to drop, but this is not useful information at that point since the usable propane is gone. The weight of the tank is the only valid measure of fullness.
Table 1 - Pressure - Temperature Chart
| psig | Temperature ° F | ||||
|---|---|---|---|---|---|
| R-22 | R-134a | R-507A | R-290* | ||
| 0 | -41 | -15 | -52 | -43.75 | |
| 10 | -20 | 7 | -32 | -18 | |
| 20 | -5 | 22 | -17 | -5 | |
| 35 | 12 | 40 | 0 | 15 | |
| *R-290 is Propane | |||||
If liquid is not present in the vessel, the direct pressure-temperature relationship is no longer valid.
The saturation, or pressure-temperature relationship, is fixed for each fluid. In refrigeration and air conditioning, charts are readily available for all common refrigerants. At saturation, the fluid is evaporating and condensing at the same time and rate; so there is no net change in heat or temperature of the fluid. If the evaporating molecules are allowed to move away from the closed chamber, they will take heat along with them, if more molecules are forced into the vessel, they will bring heat to the liquid as they condense.
This pressure-temperature or temperature-pressure relationship is the basis of all air conditioning and refrigeration. By controlling the pressure of a refrigerant, the boiling temperature of the refrigerant can be selected. It is strange to think of boiling at a cold temperature, but refrigerants routinely boil at temperatures below 0°F. Table 2 illustrates this characteristic. Note that this chart relates pressure to temperature while Table 1 relates temperature to pressure. The data is the same, only the way of showing it is different.
Table 2 - Temperature - Pressure Chart
| Temperature ° F | psig | ||||
|---|---|---|---|---|---|
| R-22 | R-134a | R-507A | R-290* | ||
| -20 | 10.2 | 3.7 InHg | 18 | 11 | |
| 0 | 24 | 14.2 | 35.3 | 23.9 | |
| 20 | 43.1 | 18.4 | 58.8 | 41.1 | |
| 40 | 68.6 | 35 | 89.9 | 63.9 | |
| 60 | 102 | 57.4 | 129.9 | 93 | |
| *R-290 is Propane | |||||
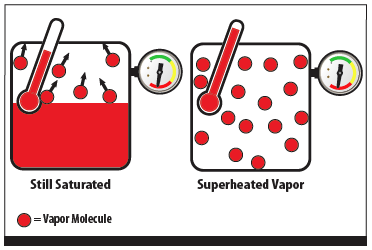
Superheat occurs when heat is added after all the liquid in a closed vessel or system has evaporated. Once all the liquid is gone, additional heat will raise the temperature of the gas, without raising its pressure. Since there is no liquid present at the same time, the fluid is not saturated and the pressure-temperature relationship of saturation no longer applies.
Superheat is a condition where the vapor is warmer than the saturated temperature at a given pressure, so by definition, saturation is zero superheat. Unfortunately, when a system is at 0 degrees of superheat, liquid is present but there is no way to know how much. Compressors cannot compress liquid, so it is important that systems only provide superheated vapor to the compressor.
Figure 2 - Saturation vs. Superheated Vapor
An azeotrope is a mixture of liquids that maintain their relative ratios even during boiling and evaporation. As a result, they are also called constant boiling mixtures. Even though each of the components may have a different boiling point, the mixture will always boil at the same temperature at a given pressure. Simply put, azeotropes act as a single component and the saturation curve has a unique pressure-temperature relationship. In Table 1 and 2; R-22, R-134a and propane are all single components and not mixtures of liquids. R-507A is a mixture of two refrigerants (50/50 mix of R-125 and R-143a) but acts as a single component under most conditions.
Zeotropic refrigerants are mixtures in which the components cause the boiling point and condensing point to occur at slightly different temperatures. They are also subject to fractional distillation or fractionation. This means that each component can boil out of the mixture separately and affect the ratio of the components in the remaining mixture. In both the liquid and vapor phase, the mixture will perform as a single fluid, but during evaporation and condensing, the components will act independently. Charts for these refrigerants are not strictly “saturation” charts, but instead show “bubble” and “dew” point.
Glide is a term used with zeotropic and near azeotropic blends and refers to the fact that each component will boil and condense at different temperatures, at a common pressure.
Table 3 - Zeotropic Refrigerants Chart
| R-401A | |||
|---|---|---|---|
| psig | Dew Point- Evaporating | Bubble Point- Condensing | Glide |
| 15.3 | -22.2 | -21.02 | 1.2 |
| 25.3 | -9.12 | -8.01 | 1.1 |
| 35.3 | 1.65 | 2.71 | 1.06 |
| 205 | 90.27 | 90.94 | .67 |
| 245.3 | 102.48 | 103.09 | .61 |
| 285.3 | 113.34 | 113.9 | .56 |
Subcooling is almost the opposite of superheat. It is a measure of how much cooler than saturation the liquid is at a given pressure. As refrigerant condenses in the condenser, it becomes saturated because both liquid and vapor are present in the same place. If the liquid remained at saturation as it moved down the liquid line, any pressure drop would cause it to reevaporate.
The re-evaporated vapor is called “flash gas” and will affect the operation and efficiency of the valves and evaporator. By subcooling the liquid, small amounts of pressure drop will not cause flash gas formation. Depending on how the subcooling is accomplished, overall system efficiency can be improved with additional subcooling. It is important to remember that subcooling does not imply that the liquid is cold, only that is it below the saturation temperature of the refrigerant.
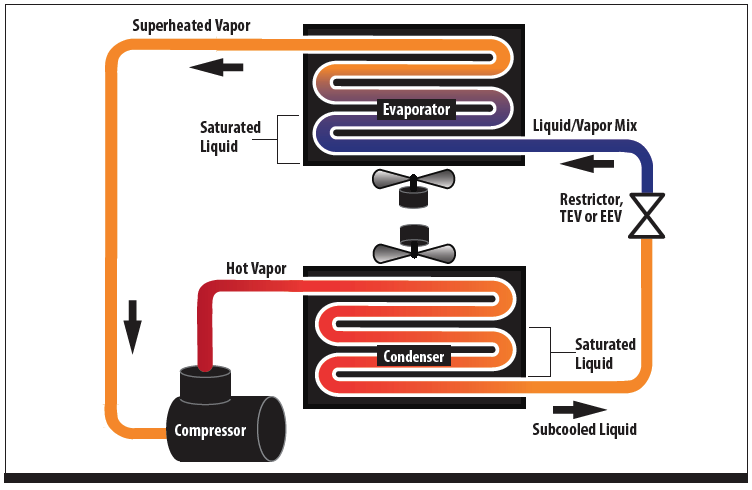
Putting it together into a system is shown in Figure 3. The basic refrigeration and air conditioning system is composed of an evaporator, condenser, compressor and expansion device. In both the evaporator and condenser, refrigerant is changing state and will pass through a saturated condition. In the condenser, a superheated vapor is cooled and becomes a saturated vapor which then condenses into a saturated liquid. Further cooling of the liquid will cause it to become subcooled.
The opposite occurs in the evaporator; a saturated liquid absorbs heat and boils into a superheated vapor, of course, at a much lower temperature. The vapor returns to the compressor to complete the cycle.
The opposite occurs in the evaporator; a saturated liquid absorbs heat and boils into a superheated vapor, of course, at a much lower temperature. The vapor returns to the compressor to complete the cycle.
Figure 3 - Basic Vapor Compression Cycle
Questions
- If liquid and vapor are together in a closed vessel, is the fluid saturated?
- Does an azeotrope behave as separate components or as a single fluid?
- Should the fluid returning to the compressor be superheated, saturated or subcooled.
- If R-22 is allowed to evaporate at 20 psig, what will be its temperature?
Going Further
An in depth explanation of refrigerant behavior is available at: http://www.refrigerants.com/ReferenceGuide2006.pdf
The standards body for Heating, Air Conditioning and Refrigeration is: http://www.ashrae.org
ASHRAE is a professional society and the handbooks are available only with membership, but many libraries have copies. Older versions of the handbooks may be available, from ASHRAE members, for the asking.
